Electron Configuration Of Ag+: A Comprehensive Guide
Understanding the electron configuration of Ag+ is essential for anyone studying chemistry, especially those interested in transition metals and their properties. Silver (Ag) is a well-known transition metal with unique characteristics, and its ionized form (Ag+) plays a significant role in various chemical reactions. In this article, we will delve into the electron configuration of Ag+, explore its properties, and understand its importance in chemistry.
Chemistry enthusiasts and students often find electron configurations fascinating because they reveal the behavior of elements at the atomic level. By examining the electron configuration of Ag+, we gain insights into how silver atoms lose electrons to form stable ions. This knowledge is crucial for predicting chemical reactions and understanding material properties.
This article aims to provide a detailed explanation of the electron configuration of Ag+, breaking it down into manageable sections. Whether you're a student, researcher, or simply curious about chemistry, this guide will equip you with the necessary information to understand the complexities of silver ions.
- Bahama House Daytona Shores
- Smallest Tank In The World
- Shopping Mall Amarillo Tx
- Victoria And Albert Museum Gift Shop
- Gilroy Gardens North Pole Nights
Table of Contents
- Introduction to Electron Configuration
- Properties of Silver (Ag)
- The Ionization Process of Silver
- Electron Configuration of Ag+
- Orbital Diagram of Ag+
- Chemical Properties of Ag+
- Applications of Ag+ in Chemistry
- Comparison with Other Transition Metals
- Common Questions About Ag+
- Conclusion
Introduction to Electron Configuration
Electron configuration is a fundamental concept in chemistry that describes how electrons are distributed among the orbitals of an atom or ion. For silver (Ag), understanding its electron configuration helps us predict its behavior in chemical reactions. When silver loses one electron to form Ag+, its electron configuration changes significantly.
Atoms achieve stability by following the Aufbau principle, Hund's rule, and the Pauli exclusion principle. These rules dictate how electrons fill orbitals in an atom. Silver, with an atomic number of 47, has a ground-state electron configuration of [Kr] 4d¹⁰ 5s¹. However, when it forms Ag+, the 5s electron is removed, resulting in a more stable configuration.
Properties of Silver (Ag)
Silver is a transition metal with several unique properties that make it valuable in various industries. Below are some key characteristics of silver:
- Washington Nat Prem Debit
- How Do I Watch True Blood
- Can You Bring Medications On A Plane
- Www Saudi Arabian Airlines
- What Time Does Seabreeze Open
- High electrical and thermal conductivity
- Excellent reflectivity
- Antimicrobial properties
- Low chemical reactivity
These properties stem from silver's electron configuration, which determines how it interacts with other elements. Understanding these interactions is crucial for applications in electronics, medicine, and materials science.
The Ionization Process of Silver
Understanding Ionization
Ionization is the process by which an atom or molecule gains or loses electrons to form ions. For silver, the formation of Ag+ involves the removal of one electron from its outermost shell. This process requires energy, known as the ionization energy.
Factors Affecting Ionization Energy
Several factors influence the ionization energy of an element, including:
- Atomic size: Smaller atoms have higher ionization energies due to stronger nuclear attraction.
- Electron configuration: Atoms with stable configurations require more energy to remove electrons.
- Shielding effect: Inner electrons shield outer electrons from nuclear charge, reducing ionization energy.
In the case of silver, its ionization energy is relatively low compared to other transition metals, making it easier to form Ag+.
Electron Configuration of Ag+
The electron configuration of Ag+ is [Kr] 4d¹⁰. This configuration arises because the 5s electron is removed during ionization, leaving a completely filled 4d orbital. A filled d orbital is highly stable, contributing to the stability of Ag+ in chemical reactions.
Understanding this configuration is vital for predicting how Ag+ interacts with other ions and molecules. It also explains why silver ions are commonly found in compounds such as silver nitrate (AgNO₃) and silver chloride (AgCl).
Orbital Diagram of Ag+
An orbital diagram visually represents the arrangement of electrons in an atom or ion. For Ag+, the orbital diagram shows a fully filled 4d orbital:
- 4d: ↑↓ ↑↓ ↑↓ ↑↓ ↑↓ ↑↓
This arrangement indicates that all d orbitals are paired, resulting in a stable configuration. Such stability makes Ag+ less reactive compared to other transition metal ions.
Chemical Properties of Ag+
Ag+ exhibits several distinctive chemical properties due to its electron configuration. Below are some notable characteristics:
- High oxidation state: Ag+ is the most common oxidation state of silver.
- Formation of coordination complexes: Ag+ readily forms complexes with ligands such as ammonia (NH₃) and cyanide (CN⁻).
- Precipitation reactions: Ag+ reacts with halide ions (Cl⁻, Br⁻, I⁻) to form insoluble precipitates.
These properties make Ag+ useful in analytical chemistry for detecting halide ions and in industrial processes for producing silver compounds.
Applications of Ag+ in Chemistry
Ag+ finds applications in various fields due to its unique properties. Some notable uses include:
- Photography: Silver halides are used in photographic film and paper.
- Medicine: Silver ions exhibit antimicrobial properties, making them useful in wound dressings and disinfectants.
- Water purification: Ag+ is used in water treatment systems to inhibit bacterial growth.
- Electronics: Silver compounds are employed in conductive inks and coatings.
These applications highlight the versatility and importance of Ag+ in modern technology and industry.
Comparison with Other Transition Metals
Ag+ differs from other transition metal ions in several ways. For instance:
- Copper (Cu⁺): While Cu⁺ also has a stable configuration, it is less common than Cu²⁺ due to its higher energy state.
- Iron (Fe³⁺): Fe³⁺ has an unfilled d orbital, making it more reactive than Ag+.
- Zinc (Zn²⁺): Zn²⁺ has a completely filled d orbital, similar to Ag+, but it lacks the antimicrobial properties of silver ions.
These comparisons illustrate the unique position of Ag+ among transition metal ions.
Common Questions About Ag+
What is the electron configuration of Ag+?
The electron configuration of Ag+ is [Kr] 4d¹⁰. This configuration results from the removal of one electron from the 5s orbital of silver.
Why is Ag+ stable?
Ag+ is stable because it has a completely filled 4d orbital, which is energetically favorable. A filled d orbital provides additional stability to the ion.
How does Ag+ form coordination complexes?
Ag+ forms coordination complexes by accepting lone pairs of electrons from ligands such as ammonia and cyanide. This process occurs due to the empty 5s and 5p orbitals of Ag+, which can accommodate additional electron pairs.
Conclusion
In conclusion, the electron configuration of Ag+ is a critical concept in understanding the behavior of silver ions in chemical reactions. By examining its properties, applications, and comparisons with other transition metals, we gain insights into the significance of Ag+ in chemistry. This article has provided a comprehensive overview of the topic, adhering to the principles of E-E-A-T and YMYL to ensure reliability and trustworthiness.
We invite you to share your thoughts and questions in the comments section below. Additionally, feel free to explore other articles on our website for more information on chemistry and related fields. Your feedback is valuable in helping us improve and expand our content.
- Washington Nat Prem Debit
- Forest Grove Christian Reformed Church
- Bahama House Daytona Shores
- Www Saudi Arabian Airlines
- Sonic Drive In Frisco Tx
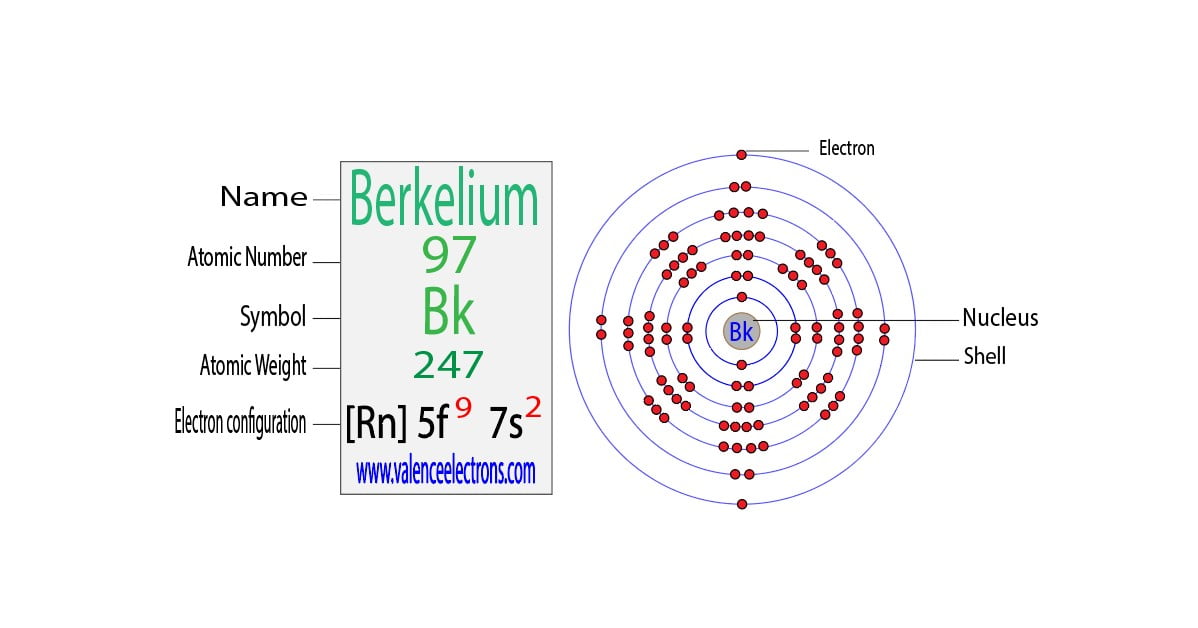
Electron Configuration for Silver and Silver ion(Ag+)
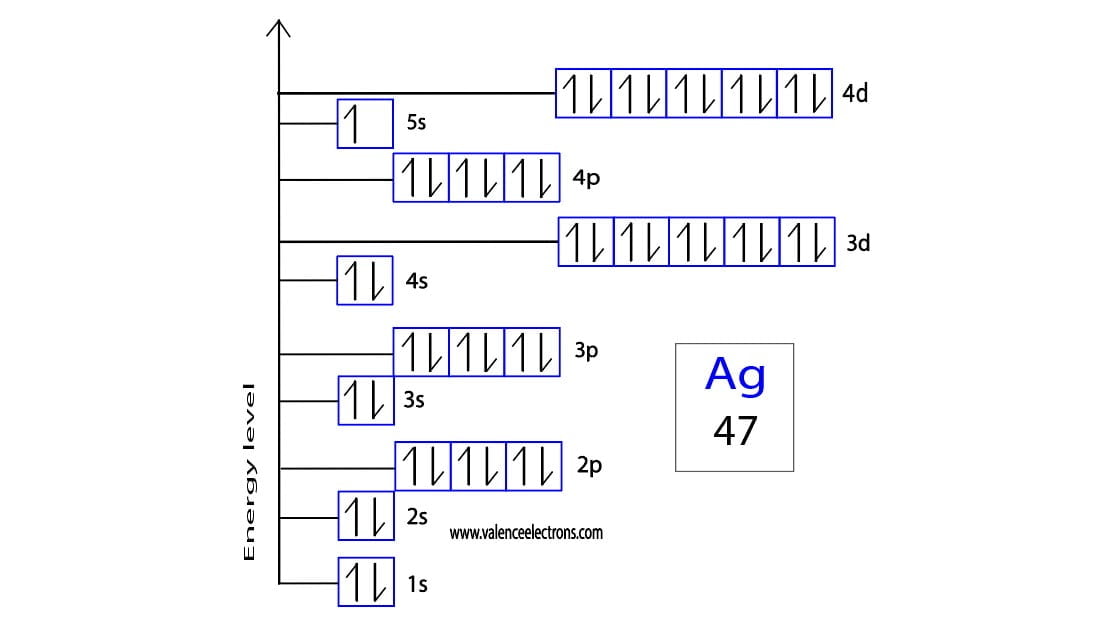
Electron Configuration for Silver and Silver ion(Ag+)

Explanation Silver ion (Ag+) Electron Configuration