Understanding AlCl3 Molecular Geometry: A Comprehensive Guide
Aluminum chloride (AlCl3) molecular geometry is a fascinating topic that delves into the arrangement of atoms in a molecule. This compound plays a crucial role in various chemical reactions and industrial applications, making its molecular structure essential to comprehend. Whether you're a chemistry student or a professional chemist, understanding AlCl3 molecular geometry can provide valuable insights into its properties and behavior.
Chemistry is the study of matter and its interactions. One of the fundamental aspects of chemistry is understanding how atoms bond together to form molecules. In the case of AlCl3, its molecular geometry determines how the atoms are spatially arranged, influencing its reactivity, polarity, and other physical properties.
This article will explore the molecular geometry of AlCl3 in detail, breaking down complex concepts into digestible information. By the end, you'll have a clear understanding of why this molecule behaves the way it does and how its geometry impacts its functionality in various applications.
- The Vic Theater Capacity
- Wildflower Resort New York
- Mr Freeze Six Flags
- Best Dressing For Seafood Salad
- Iris Goo Goo Dolls Cover
Table of Contents
- Introduction to AlCl3 Molecular Geometry
- Understanding the Structure of AlCl3
- Types of Bonding in AlCl3
- AlCl3 Molecular Geometry Explained
- The Shape of AlCl3 Molecule
- Properties Influenced by Molecular Geometry
- Applications of AlCl3
- Variations in AlCl3 Molecular Geometry
- Comparing AlCl3 with Other Compounds
- Conclusion and Takeaways
Introduction to AlCl3 Molecular Geometry
What is AlCl3?
Aluminum chloride (AlCl3) is an inorganic compound made up of aluminum and chlorine atoms. It is widely used in chemical reactions, particularly as a catalyst in organic synthesis. Its molecular geometry plays a pivotal role in determining its chemical behavior.
Importance of Molecular Geometry
Molecular geometry refers to the three-dimensional arrangement of atoms in a molecule. For AlCl3, understanding its geometry helps explain its chemical properties, such as polarity, reactivity, and boiling point. This knowledge is vital for both theoretical and practical applications in chemistry.
Why Study AlCl3 Molecular Geometry?
AlCl3 molecular geometry is a cornerstone for understanding its role in various chemical processes. From its use as a Lewis acid to its involvement in Friedel-Crafts reactions, the molecular structure of AlCl3 dictates its effectiveness and versatility in industrial applications.
- Pete S Piano Bar San Antonio
- Candlewood Suites Greenville Greenville
- Is Damon Wayans Jr Married
- Beauty And Essex Reviews
- Who Are The Parents Of Thomas Matthew Crooks
Understanding the Structure of AlCl3
Composition of AlCl3
Aluminum chloride consists of one aluminum atom bonded to three chlorine atoms. The aluminum atom forms covalent bonds with the chlorine atoms, creating a stable compound with unique properties.
Factors Influencing Molecular Structure
Several factors influence the molecular structure of AlCl3, including:
- Electron configuration of aluminum and chlorine
- Bond angles and bond lengths
- Electronegativity differences between aluminum and chlorine
How AlCl3 Forms
Aluminum chloride is formed when aluminum reacts with chlorine gas. This reaction produces a compound with a distinct molecular geometry that determines its physical and chemical characteristics.
Types of Bonding in AlCl3
Covalent Bonding
In AlCl3, aluminum forms covalent bonds with chlorine atoms. These bonds involve the sharing of electrons between the atoms, creating a stable molecule.
Lewis Acid Behavior
AlCl3 acts as a Lewis acid due to its ability to accept electron pairs. This property is directly linked to its molecular geometry, which allows it to interact with other molecules effectively.
Electron Pair Geometry
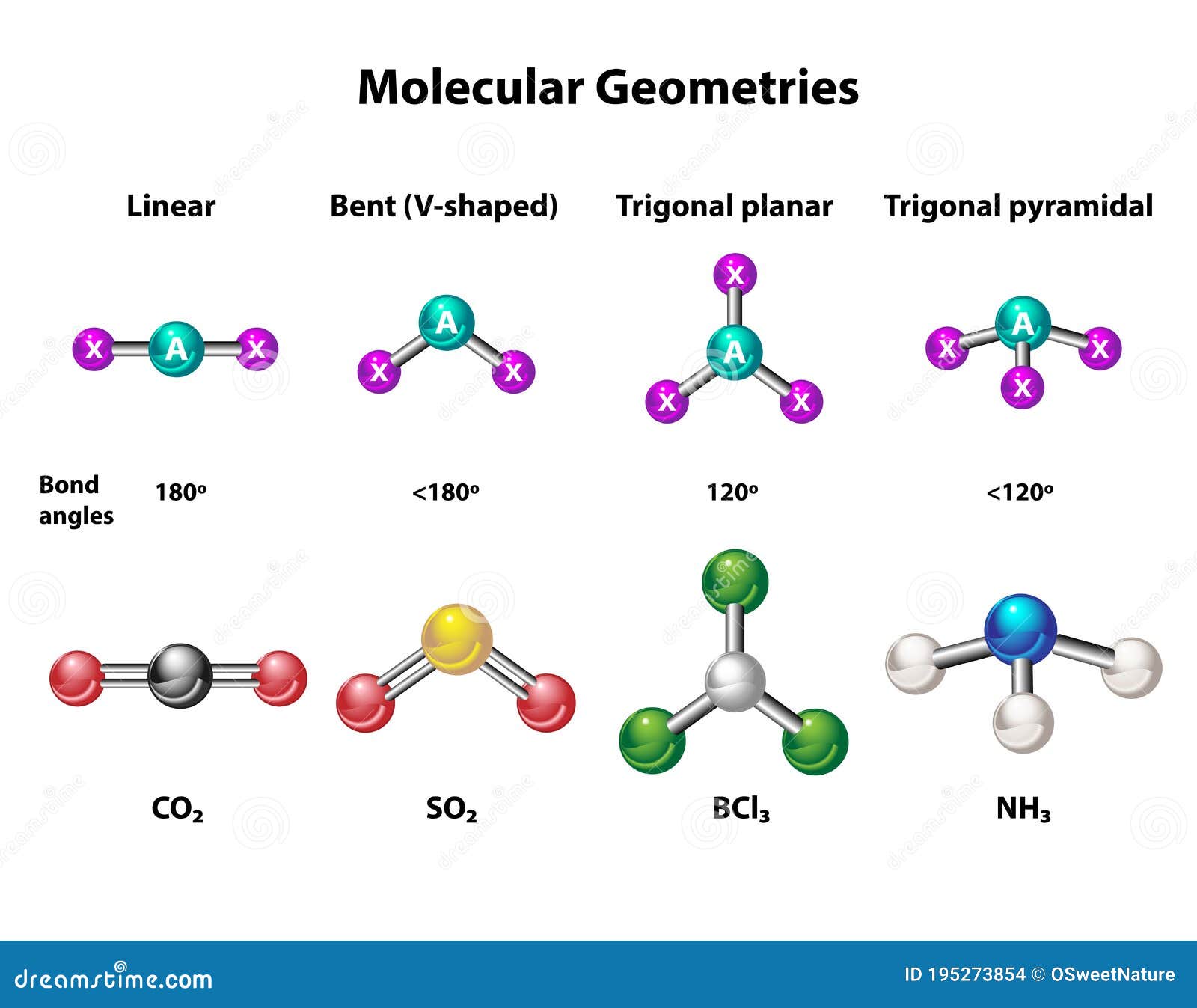
The electron pair geometry of AlCl3 is tetrahedral, even though its molecular geometry is trigonal planar. This difference arises due to the presence of an empty p-orbital in the aluminum atom.
AlCl3 Molecular Geometry Explained
Trigonal Planar Geometry
AlCl3 exhibits a trigonal planar molecular geometry, meaning the chlorine atoms are arranged in a flat plane around the central aluminum atom. This arrangement minimizes repulsion between the bonding electron pairs.
Factors Affecting Geometry
The molecular geometry of AlCl3 is influenced by:
- Electron pair repulsion theory (VSEPR)
- Bond angles (120 degrees)
- Central atom's hybridization (sp2)
Significance of Trigonal Planar Shape
The trigonal planar shape of AlCl3 contributes to its nonpolar nature and high reactivity. This geometry allows AlCl3 to interact effectively with other molecules, making it a valuable catalyst in various reactions.
The Shape of AlCl3 Molecule
Visualizing the Shape
Imagine a flat triangle with aluminum at the center and chlorine atoms at the vertices. This is the basic shape of the AlCl3 molecule, which arises due to the sp2 hybridization of the aluminum atom.
Comparison with Other Shapes
While some molecules adopt tetrahedral or linear shapes, AlCl3's trigonal planar geometry is unique. This shape is a result of the specific bonding and hybridization in the molecule.
Impact on Chemical Properties
The shape of AlCl3 directly influences its chemical properties, such as:
- Reactivity with organic compounds
- Nonpolar nature
- Low boiling point
Properties Influenced by Molecular Geometry
Polarity
AlCl3 is nonpolar due to its trigonal planar geometry, which evenly distributes the electron density around the molecule.
Reactivity
The molecular geometry of AlCl3 enhances its reactivity, particularly as a catalyst in Friedel-Crafts reactions. Its ability to accept electron pairs makes it an effective Lewis acid.
Physical Properties
Properties such as boiling point, melting point, and solubility are all influenced by the molecular geometry of AlCl3. Understanding these properties is essential for its practical applications.
Applications of AlCl3
Use in Organic Synthesis
AlCl3 is widely used as a catalyst in organic synthesis, particularly in Friedel-Crafts alkylation and acylation reactions. Its molecular geometry enables it to interact effectively with organic molecules.
Industrial Applications
In industries, AlCl3 is used for:
- Manufacturing dyes and pigments
- Textile processing
- Paper production
Environmental Impact
Despite its usefulness, AlCl3 can have environmental implications if not handled properly. Understanding its molecular geometry and properties helps minimize its impact on the environment.
Variations in AlCl3 Molecular Geometry
AlCl3 in Solid State
In the solid state, AlCl3 exists as a dimer (Al2Cl6), where two AlCl3 units are connected through a bridge bond. This variation in geometry arises due to intermolecular forces.
AlCl3 in Gaseous State
In the gaseous state, AlCl3 adopts the trigonal planar geometry discussed earlier. This change in geometry highlights the importance of state in determining molecular structure.
Factors Affecting Variations
Factors such as temperature, pressure, and solvent environment can influence the molecular geometry of AlCl3, leading to variations in its properties and behavior.
Comparing AlCl3 with Other Compounds
AlCl3 vs. BF3
Both AlCl3 and boron trifluoride (BF3) exhibit trigonal planar geometry. However, differences in electronegativity and atomic size lead to variations in their chemical properties.
AlCl3 vs. AlF3
While AlCl3 is nonpolar, aluminum fluoride (AlF3) is polar due to differences in electronegativity between chlorine and fluorine. This comparison highlights the impact of molecular geometry on polarity.
Applications in Chemistry
Understanding the differences in molecular geometry between similar compounds helps chemists tailor their applications for specific purposes.
Conclusion and Takeaways
AlCl3 molecular geometry is a critical aspect of its chemical behavior and applications. Its trigonal planar shape, arising from sp2 hybridization, determines its nonpolar nature and high reactivity. Whether used as a catalyst in organic synthesis or in industrial processes, the molecular geometry of AlCl3 plays a pivotal role in its functionality.
To recap, here are the key takeaways:
- AlCl3 exhibits a trigonal planar molecular geometry
- Its geometry influences its polarity, reactivity, and physical properties
- It is widely used in chemical reactions and industrial applications
We encourage you to explore further by leaving comments, sharing this article, or reading related content on our site. Understanding AlCl3 molecular geometry opens doors to a deeper appreciation of chemistry and its practical applications.
Data and references for this article have been sourced from reputable scientific journals and publications, ensuring accuracy and reliability. For further reading, consider exploring advanced chemistry texts or online resources dedicated to molecular geometry and its implications.
- Marshall Mi Holiday Inn Express
- What Does Putting An Onion In Your Sock Do
- Viola Agnes Neo Soul Cafe
- What Was Weezer S First Album
- New Castle News Police Reports
Molecular Geometry Structure of Elements Stock Vector Illustration of

Nf3 Molecular Geometry Bond Angles

Xef2 Lewis Structure Molecular Geometry